Background:
Immunomodulatory drugs (IMiDs) are the current backbone of standard and experimental combination myeloma therapies at all stages of disease, but the majority of patients eventually relapse. The mechanisms driving IMiD resistance are poorly understood. Previous studies looking for genetic drivers of resistance have looked at core members of the CRL4CRBN E3-ubiquitin ligase complex (CUL4-RBX1-DDB1-CRBN) and identified infrequent mutations and deletions in cereblon (CRBN), but at a rate that cannot account for resistance in the majority of patients. More recently several in vitro studies have identified novel regulators of cereblon activity including the COP9 signalosome, E2 ubiquitin conjugating enzymes, neddylation modifiers and additional IMiD neosubstrates.
In this study paired presentation/relapse samples from newly diagnosed patients recruited to a clinical trial (UK NCRI Myeloma XI trial: NCT01554852) of largely IMiD-based therapies were used to investigate the role of mutations and deletions in all genes implicated in IMiD activity. For comparison, cell line models of resistance were generated in vitro.
Methods:
56 patients who received IMiD induction therapy followed by either lenalidomide maintenance (n=30) or observation (n=26), and subsequently relapsed, underwent whole exome sequencing (WES) of CD138+ cells, median depth 122x for tumour samples and 58x for paired germline controls. Non-synonymous mutations and deletions present in tumour but not germline controls were considered.
Cell line models were generated using the IMiD sensitive MM1s cell line. Cells were cultured in 10xGI50 concentrations of lenalidomide/pomalidomide alongside a control exposed to the same %DMSO. WES was carried out and non-synonymous mutations identified. Mutations present in the lenalidomide resistant (Len-R) and pomalidomide resistant (Pom-R) but not their relevant DMSO exposed control were considered.
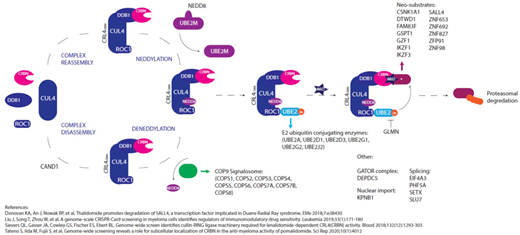
From recent publications a list of 42 genes (Figure 1) involved in cereblon pathway regulation and IMiD response was curated, termed "CRBN/IMiD genes". Mutations in CRBN/IMiD genes in the patient dataset and cell line models were examined.
Results:
In the patient data set 12/42 (28.6%) of the CRBN/IMiD genes were found to be mutated, with a total of 17 mutations in 14/56 (25%) patients identified. 9/17 (53%) were identified in patients who had received lenalidomide maintenance and 8/17 (47%) in the observation group. Importantly, in the patients receiving lenalidomide maintenance, 6 of the 9 (66.7%) mutations had a higher cancer clonal fraction (CCF) at relapse, suggesting they may have been selected for by exposure to treatment. Comparatively, in mutations identified in patients undergoing observation, only 3 of the 8 (37.5%) mutations had a higher CCF at relapse compared with presentation. The only deletion in CRBN/IMiD genes was in SETX, in one patient at relapse.
Only one mutation or deletion was identified in CRBN itself, a missense mutation at relapse at g.3:3195148A>C, encoding a Cys326Gly sequence modification at the protein level. Interestingly, Cys326 is one of 4 cysteines in CRBN coordinating a single zinc ion to form a Zn finger motif, which stabilises the Thalidomide Binding Domain (TBD) of the protein, suggesting this mutation may have had functional significance.
In the cell line models full resistance up to 100xGI50 concentrations was established by 12 weeks. The resistant cell lines had cross-resistance to the other IMiDs and comparable morphology, growth rates and responses to non-IMiD drugs as their sensitive counterpart. Resistant cells had reduced levels of CRBN mRNA and protein expression. Functional assays demonstrated that well characterised downstream effects of IMiD treatment were abrogated: transcription factors Ikaros and Aiolos not degraded and no downregulation of IRF4 mRNA. The Pom-R cell line had a mutation affecting a CRBN splice site 5' of exon 8. No other mutations or deletions in the 42 IMiD pathway genes were identified in either the Len-R or Pom-R lines.
Conclusions:
CRBN and other genes in the IMiD response pathway were mutated or deleted in around 25% of patients suggesting other mechanisms, for example epigenetic alterations, underlie resistance acquisition in a significant proportion. Models for both CRBN/IMiD gene mutated and unmutated resistant states have been generated and will be used to study mechanisms of IMiD resistance.
Jones:Celgene: Honoraria, Research Funding. Che:Monte Rosa Therapeutics: Research Funding. Le Bihan:Monte Rosa Therapeutics: Research Funding. Wang:Monte Rosa Therapeutics: Research Funding. Kaiser:Bristol-Myers Squibb, Chugai, Janssen, Amgen, Takeda, Celgene, AbbVie, Karyopharm, GlaxoSmithKline: Consultancy; Janssen, Amgen, Celgene, Bristol-Myers Squibb, Takeda: Honoraria; Bristol-Myers Squibb/Celgene, Janssen, Karyopharm: Research Funding; Bristol-Myers Squibb, Takeda: Other: Travel expenses. Jackson:Takeda: Honoraria, Research Funding, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Gsk: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding, Speakers Bureau. Davies:Celgene/BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotech: Honoraria; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees. Chopra:Apple Tree Life Sciences: Current Employment; Monte Rosa Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Research Funding. Morgan:GSK: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Janssen: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Pawlyn:Takeda: Consultancy, Other: Travel expenses; Celgene: Consultancy, Honoraria, Other: Travel expenses; Janssen: Honoraria, Other: Travel expenses; Amgen: Consultancy, Other: Travel expenses.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal